A novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 in Wuhan, Hubei Province, China.1 Since then the disease has spread to many countries and was declared a public health emergency of international concern (PHEIC) by the World health organization on 30 January 2020.2 Prior to this time, 6 coronavirus species were known to cause human disease, four of which is known to cause common cold-like infections in immunecompetent humans (229E,OC43,HKU1,NL63), the other two namely, severe acute respiratory syndrome coronavirus(SARS-COV) and the Middle East respiratory syndrome coronavirus (MERS-CoV), are zoonotic in origin and are known to cause severe respiratory disease in humans. A lot is known about the disease whose common symptoms are fever, cough and shortness of breath. Globally, as at 8th September 2020, there have been 27,236,916 confirmed cases of COVID-19, including 891,031 deaths, reported to WHO with increasing numbers everyday.3
In spite of all efforts to contain the pandemic, the number of infections and deaths from COVID-19 keeps increasing. Elderly persons and adults with other background medical conditions have been the worst affected.4 Many documented studies on COVID-19 are mostly on adults and some on older children with few on neonates.
In Italy, Japan, and Thailand, there were community based infections with cases of SARS-CoV-2 associated pneumonia reported in children.4–6 Reported paediatric cases were mainly family cluster cases with epidemiological links to adults.4–6 Peadiatric cases appear to have a milder clinical course than those in adults and rarely deaths have been reported in children.6 These may imply that pediatric cases may play a role in community spread of SARS-CoV-2 since the viral shedding may continue from nasal secretions as well as through fecal matter for several weeks after diagnosis.7 Infections of SARS-CoV-2 in neonates have been said to be mild also.7 The reason behind the less susceptibility and mild infections of SARS-CoV-2 in neonates is largely unclear especially as neonates are not generally protected from respiratory viral infections. Although vertical transmission of COVID-19 is still being debated, maternal COVID19 infections especially when associated with severe hypoxia and fever may result in fetal distress, premature delivery and other risks.
Limited evidence exists however on mode of transmission, prevalence and clinical features of COVID-19 in neonates. It has been shown that mothers may transmit the infections by droplets during breastfeeding or by vertical transmission.8 Common presentations of fever and respiratory signs have been found to occur in neonates with COVID-19 however other existing co- morbidities such as prematurity and chromosomal disorder makes it unclear as to the actual cause of these signs.1,9 Other times, neonates may present with non-specific symptoms such as lethargy and dehydration making diagnosis difficult. Treatment of COVID-19 have been largely symptomatic with no definitive therapy.3,7 Drug trials have been largely done in adult population. A clear understanding of the symptomatology of COVID-19 among neonates, its mode of transmission and diagnosis is needed to improve public health and clinical response. There is some knowledge gap with regards to COVID-19 in neonates despite its epidemiological importance, and therefore, it is our objective to describe the documented modes of transmission, clinical features, treatment, complications and outcome of infections of SARS-CoV-2 in neonates by a systematic review of existing literature.
METHODS
The Preferred Reporting Items for systematic Reviews and Meta-Analysis (PRISMA) checklist for reporting a systematic review or meta-analysis protocol was used for this review (Online Supplementary Document, Appendix 1).10
Search strategy and selection criteria
A systematic search for literature was conducted on 5th April 2020 and on 4th August 2020. The databases searched include PubMed, Ovid MEDLINE, EMBASE, CINAHL, Web of Science, and Google Scholar. Other COVID-19 data provided freely by various institutions on their websites were also retrieved. The search terms used included keywords, text words and medical subject headings (MeSH) terms and subheadings of the following: COVID-19, SARS-CoV-2, Corona virus, Newborn, Neonate, exposure, vertical transmission, breastfeeding, droplet infection.
Inclusion and exclusion criteria
We included primary studies that were carried out globally either in hospitals or in the communities such as case reports, case series, cross-sectional studies and other qualitative or quantitative studies and published in English language, between January and August 2020 which reported COVID-19 exposure, transmission, infection, and disease in the newborn.. Review articles and studies in other languages apart from English were excluded.
Study selection
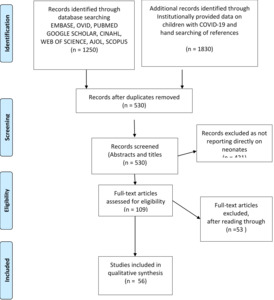
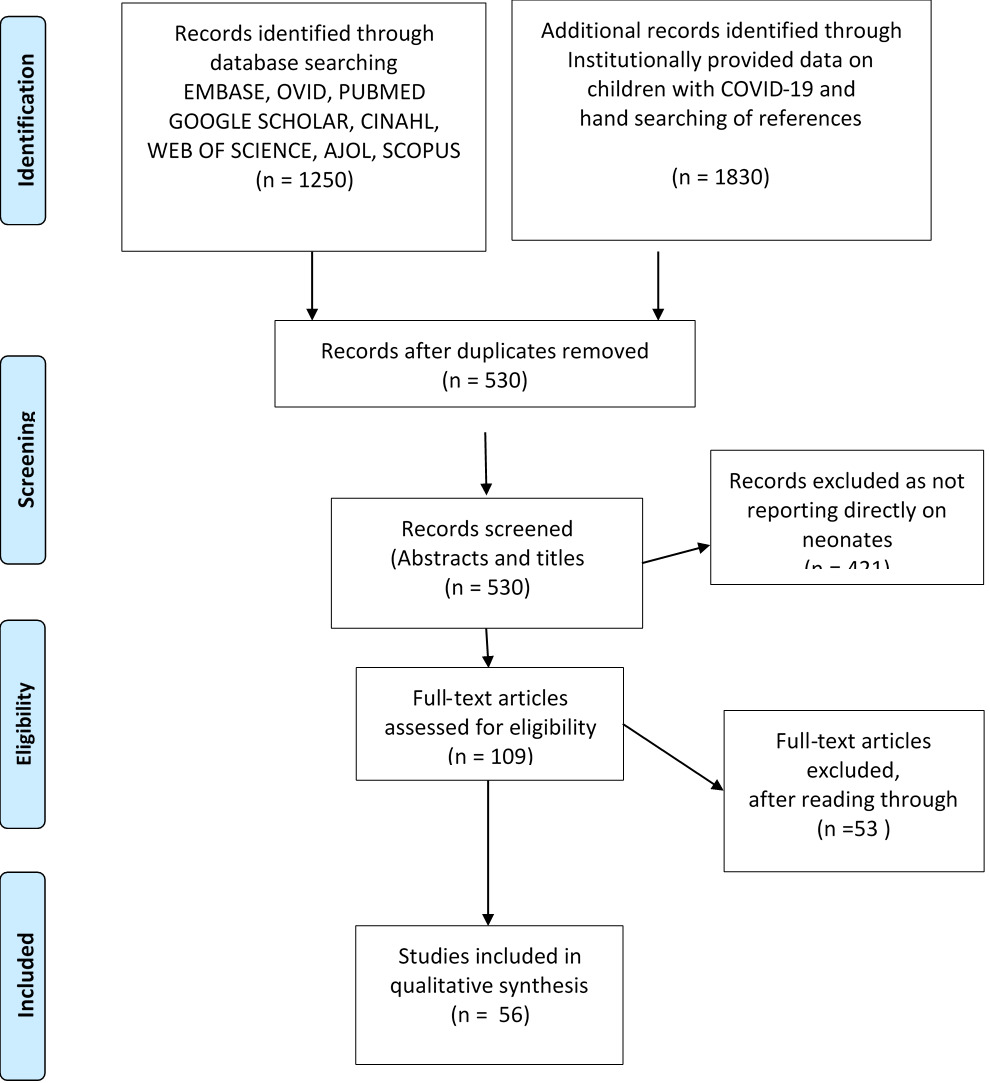
A total 1250 studies were identified from the initial search of databases and institutional sources 1830 from other sources including institutionally provided data. After duplicates were removed, the number of remaining publications was 530 articles, and these were reviewed for inclusion based on information contained in titles and abstracts.
Studies not addressing exposure of transmission to or infection in newborns were excluded to give a total of 109 full text articles. These were assessed further, and 56 full text articles were included for the review (Figure 1).
Data extraction
A data extraction form was developed and reviewed by all authors. Data was extracted for each paper using the standardised form with the following domains: the name of first author and year of publication, study location, study design, clinical features, laboratory and radiological findings, diagnosis of Covid-19, treatment and outcome. Two of the reviewers extracted the data independently and discrepancies were resolved by discussion and consensus. The references were tracked using EndNote reference manager where duplicates were also removed.
Quality assessment of studies
Quality assessment of studies was done using the Newcastle Ottawa Scale for Case Control Studies,11 Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies,12 and the Tool for Evaluating the Methodological Quality of Case Reports and Case Series13 (Online Supplementary Document, Appendix 2). All the case reports and case series included in this review fulfilled the quality criteria (Online Supplementary Document, Appendix 3). For the cross sectional studies, most of the studies had “not applicable” or “cannot determine” as responses to the questions on sample size calculation, measurement of exposure and outcome variables and on statistical analysis, but fulfilled the criteria with regards to questions on study objectives, study population and sampling (Online Supplementary Document, Appendix 4).
Ethics considerations
Ethical approval was not required for this study because it is a systematic review with no direct involvement of human or animal study participants.
RESULTS
Study characteristics
Fifty-six studies were included in this review. Thirty one of the studies were conducted in China while the remaining studies were carried out in Iran (five)1,14–17, South Korea (two)2,18, USA (seven)3,19–24, Italy (four)25–28, Spain (three)29–31, Peru (one)4, France (one)32, Australia (one)33, Belgium (one)34 (Table 1). All the studies were published between January and August 2020 (Table 1). The study designs included in this review were 33 case reports, 13 case series, one expanded case series, one case control study, and eight cross-sectional studies(Table 1). From the included studies, a total of 416 neonates were examined between few hours old and 28 days of age. A total of 38 neonates had PCR-confirmed COVID-19 disease following testing (Table 2). Fourteen (36.8%) out of the 38 neonates that tested positive had no symptoms.14–16,23,25,29,34–39
Clinical and laboratory features
The following are clinical and/or laboratory examinations and results that were described by the studies.
Clinical features
- Systemic features: Thirteen studies reported neonatal fever after birth to a COVID-19 positive mother with a total of 17 neonates.1,2,17,21,22,30,35,36,38,44,46,48,54 Four studies showed that four neonates had lethargy.1,17,20,44 Swollen superficial lymph nodes was identified in a COVID-19 positive male neonate,7 and hypothermia was reported in one neonate (Table 3).3
-
Respiratory features reported include cough (four neonates),2,4,17,36 nasal discharge, congestion and stuffiness (6 neonates),1–3,17,30 tachypnoea (21 neonates),1,3,5,7,28,31,38,44–47 respiratory distress syndrome (5 neonates),42,44 mild respiratory distress (3 neonates),1,4,32 moaning (3 neonates),5,45 asphyxia (4 neonates),38,44,59 and sneezing (1 neonate) (Table 3).7
-
Cardiovascular features include tachycardia in 5 neonates, cyanosis in 12 neonates, dyspnoea in 9 neonates1,4,17,28,31,36,38,45 and hypotension in one neonate (Table 3).3
-
Skin/dermatological features reported include cutaneous mottling (one neonate),1 whole body jaundice (one neonate),2 rash (3 neonates),45,46 ruddy skin colour (2 neonates)7,8 and skin ulceration (one neonate) (Table 3).45
-
Gastrointestinal features: Abdominal distension was reported in 3 neonates,28,46,48 reduced feeding and feeding intolerance in 8 neonates,3,7,22,28,44,46 and reduced milk intake in 8 neonates.3,7,17,21,28,46,50,54 Milk refusal, bloating and gastric bleeding was reported among COVID-19 negative neonates born to positive mothers in one study.46 Nine studies reported vomiting in nine neonate2,7,8,30,36,42,44,46,54 (Table 3) while diarrhoea was reported in two neonates in two studies.7,54
Radiological and laboratory results
-
SARS-COV-2 testing following childbirth: 38 (9.0%) neonates had PCR confirmed COVID-19 infection. For majority of the babies, nasopharyngeal swab was used for testing.
-
Radiological features reported include bilateral perihilar streaking (one neonate),21 ground glass opacities (12 neonates,3,7,20,27,31,32,35,60 consolidation (3 neonates),3,17,32 patchy fuzzy shadows (one neonate),54 bilateral streaky infiltrates (one neonate),34 pneumothorax (one neonate),46 reduced lung volume(two neonates),5,20 thickened lung texture (one neonate),8 diffuse haziness (one neonate),48 mild pulmonary infection (two neonates),46,47 pneumonia (4 neonates),44,48 Respiratory distress syndrome (RDS) (4 neonates),38,42,46 Bronchovascular shadows (2 neonates),46,60 increased lung markings (3 neonates) (Table 4).36
- Other laboratory findings identified include: Elevated white blood cell (WBC) count (10 noenates),35,44,45,58 Abnormal Liver Function Test (LFT) (four neonates),8,21,46 Elevated cytokines (two neonates), 3,42,58 elevated creatine kinase (nine),8,30,39,42,44 Lymphopenia (6 neonates) (Table 4).8,14,34,48,55
Transmission
Among the 38 neonates that tested positive, vertical transmission was reported in 11 neonates, while horizontal transmission was reported in 17 babies. The route of transmission was not clear in the remaining babies (Table 5).
Treatment received
Treatment was supportive for majority of the babies. Few received antiviral (9 neonates),1,9,17,21,47 antibiotics 23 neonates),1,3,5,8,9,17,19,21,22,27,28,30,31,35,37,38,44,48 and oxygen therapy/ventilation (22 neonates) (Table 4).4,5,17,21,23,26–29,31,34,38,42,44,45,59
Outcomes and complications
The complications reported include pneumonia, pneumothorax, refractory shock, and hypoxic respiratory failure. Neonates who tested positive were discharged after they tested negative to the virus. All the COVID-19 positive babies survived (Table 2). Three of the studies reported neonatal death in 5 neonates but these babies were not tested before death.14,46,59
DISCUSSION
The review examined studies that looked at the exposure to, transmission and infection of neonates as well as clinical manifestation, laboratory findings and outcomes of neonates whose mothers had COVID-19 infection. Few neonates of mothers with COVID-19 infection in this review tested positive to the virus. This finding does not support vertical transmission as a major mode of infection of the newborn. It should be noted however that quite a number of the neonates were delivered through caesarean section and the health care workers may have observed adequate infection prevention and control measures. Some of the neonates who had positive results had close contacts with other family members diagnosed with coronavirus and possibly got the infection from person-to-person transmission.1–3 Wang et al8 reported a positive test of COVID-19 in a 36 hour-old neonate in Wuhan, China. A possibility of nosocomial infection was entertained by the authors because the initial cord blood and placenta test results were negative for severe acute respiratory syndrome corona virus 2 (SARS-CoV-2). However, the finding of positive nasopharyngeal and anal swabs among 3 out of 33 neonates whose mothers had COVID-19 pneumonia by day 2 of life by Zeng et al44 raises the possibility of vertical transmission. Duran et al61 in a systematic review of neonatal cases also did not find any evidence of vertical transmission of corona virus.
Most of the neonates who were positive for SARS-CoV-2 had mild clinical symptoms. Mild symptoms according to World Health Organisation (WHO) interim guideline is when the patient has uncomplicated upper respiratory tract viral infection with non-specific symptoms such as fever, fatigue, cough, malaise and rarely may present with diarrhea, nausea, and vomiting.62 This finding is similar to reports in older children who also presented with mild or moderate clinical symptoms.63,64 The findings of cough, tachypnea, nasal discharge in neonates who tested positive for COVID-19 observed in this review is in keeping with findings in some studies carried out among older children and adults.65–67
Lymphopenia observed among these neonates has also been reported in older paediatric age groups.68 In adults with COVID -19 infection, lymphopenia is associated with poor prognosis and is an indicator of severe disease.68–70 Possible explanations for lymphopenia include direct infection of the lymphocytes, damage to lymphoid organs, disruption of activities of cytokines resulting in apoptosis of lymphocytes.70 Fever was a significant finding in newborns delivered to COVID-19 positive mothers, despite the fact that quite a number of these babies were negative. This could be as a result of maternal cytokines transferred passively to the fetus and evoking immune response in the neonatal period. The implication of this finding is the fact that the presence of fever in a newborn during this period of COVID-19 pandemic may be a good reason to screen mothers for SARS-CoV-2 infection. In adult populations, fever was also a frequent finding alongside fatigue and cough during presentation in the hospital.65,66
Gastrointestinal symptoms appear to be a common finding in neonates who were positive for COVID-19. These have also been reported among other paediatric age groups.71 Symptoms such as reduced feeding, vomiting and diarrhea may be due to the body’s response to acute viral replication in the gastrointestinal tract. Therefore, the presence of gastrointestinal features in a neonate born to a COVID-19 positive mother or who may have been exposed to a COVID -9 positive individual, may raise a suspicion of COVID-19 infection in the neonate. SARS-COV-2 RNA has been demonstrated in stool samples of hospitalized patients with COVID-19 with some still having positive stool test even after respiratory samples have tested negative, thereby posing a risk of feco-oral transmission.72
There is currently no definitive drug treatment for COVID-19 infection. In this review, supportive therapy which includes administration of intravenous fluids, intranasal oxygen were the mainstay of treatment for COVID-19 positive neonates because many of them had mild clinical symptoms. Some of the neonates received intravenous antibiotics as a way of preventing secondary bacterial infection. A few studies documented the use of antiviral drugs like oseltamivir and acyclovir.1,9,14,18 The effect of these drugs in the clinical course of COVID-19 infection is still being evaluated. In this review most of the neonates did not receive antiviral drugs and still had good outcome. The use of oseltamivir has been reported among adults with COVID-19 infection without significant outcome or change in disease progression.65 Chloroquine and hydroxychloroquine have been demonstrated to have in vitro activity against SARS-CoV-2.73 The combination of hydroxychloroquine and azithromycin has been reported to improve clinical outcomes among adult population in France,74 though this was a retrospective study and therefore has its limitations. In a randomized control trial in Spain among adult population with mild COVID-19 infection, no benefit was observed with use of hydroxychloroquine.75 Recently the U.S Food and Drug Administration (FDA) issued emergency use authorization of remdesivir for the treatment of suspected or laboratory confirmed severe cases of COVID-19 in adult and children.76 Therefore, more clinical trials are needed to determine the effective drug treatment for COVID-19 in both adults and children.
Most of the neonates born to COVID- 19 mothers had good outcomes. This finding gives credence to the fact that the infection is mild in neonates. Since there is currently no evidence of transmission of the virus in breast milk and because of the mild illness in this age group, WHO interim guideline recommends that infants born to mothers with suspected, probable or confirmed COVID-19 should be fed according to standard infant feeding guidelines, while applying necessary precautions for infection prevention and control.62
Strengths and limitations
One of the strengths of this study is that it’s a comprehensive global review. Secondly, it included both babies that tested negative though exposed to COVID-19 positive mothers and babies that tested positive. One limitation of this review is that it included mainly case reports and case series, which do not provide high quality evidence; however, these are the major kinds of research available considering the fact that neonatal COVID-19 is a new and evolving disease.
Conclusions
The clinical manifestations of COVID-19 in neonates are mild and outcomes are better than in adult population. There is currently little evidence of vertical transmission in neonates. Given immunological susceptibility of neonates and the fact that COVID-19 is a newly emerged and evolving disease, it is important to continue in-depth research using higher quality study designs and methodologies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorship contributions
OBE, CDO: study conceptualization and design. OBE, ICA, INO, CDO, OUA, OWD, EE: data extraction, analysis and interpretation of results, manuscript drafting and approval of the final manuscript for publication.
Competing interests
The authors completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author), and declare no conflicts of interest.