Despite global effort to reduce maternal mortality, postpartum haemorrhage (PPH) is the leading cause of maternal death worldwide. PPH accounts for a large majority of obstetrical haemorrhages and severe obstetrical morbidity.1
PPH is usually defined as blood loss of at least 500 mL, 700 mL, or 1,000 ml, according to its different guidelines.1–3 At population level, the incidence of PPH is estimated about 5% of total live births—this number varying greatly between regions and obstetric centres. Severe PPH (S-PPH), currently defined as blood loss of more than 1,000 mL accounts for about 1% of total deliveries (~20% of all parturient women).1–3
Several individual risk factors for PPH and S-PPH have been reported in the literature, including non-obstetrical and obstetrical determinants.4,5 Thus, greater attention has been paid during the last decade to the medical management of S-PPH, as it may lead to further improvements with an important impact on the disease burden.
In accordance, studies have been conducted to establish recommendations for clinical practice in managing childbirth. Nevertheless, most of these studies have focused on the period of labour and did not fully account, either for non-obstetrical individual or antepartum obstetrical individual risk factors.6,7 Furthermore, the quality of care in obstetrics is a major global health concern that requires continuous improvements. In this sense, conducting regular updates of the literature on the determinants of S-PPH can help to account for the evolution of knowledge and practices, quality emergency obstetric care (EmOC) while in turn it may also contribute to mitigating maternal risks through improving the triage and treatment of parturient women.
In this framework, we provided an up-to-date summary about the determinants for S-PPH. Our first hypothesis was that certain maternal conditions (age at first parity, grand multiparity, obesity, stress, depression, etc.) which are increasing in western countries and are classically associated with some PPH determinants (pre-eclampsia, caesarean section, instrumental extractions) could contribute to an increase in the incidence of PPH. Our second hypothesis was that the lack of identification, upstream of organizational measures aimed at preventing PPH, pushed to focus efforts on individual obstetrical care, which could be hampered by the deterioration of socio-economic conditions and access to prevention, thus facilitating the risk of S-PPH at presentation. We deduced from the above the possibility that determinants of PPH upstream of childbirth could emerge, with a strong probability that those which are not very dependent on obstetrical care, could be also associated with progression from PPH to S-PPH. Our primary objective was to identify and classify the determinants of S-PPH for decision-making purposes.
METHODS
Search strategy and study selection
This systematic review was developed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Appendix S1 in the Online Supplementary Document).8
Using the terms “risk factor”, OR “determinant”, AND “post-partum haemorrhage” AND “severe”, we searched Web of Science, Scopus, Science Direct, Medline, Cochrane (Pregnancy and Childbirth Group’s Register), ClinicalTrials.gov databases, for both interventional and observational studies (randomized controlled trials; either cross-sectional, case-control or cohort studies, respectively) published in English language, between January 1, 2004 and August 31, 2018.
The full search strategy is detailed in the protocol provided in Appendix S2 of the Online Supplementary Document.
Abstracts of identified articles were screened independently by two reviewers (TS, PG) to verify whether they matched the selection criteria. Inter-observer discrepancies were resolved by arbitration of a third reviewer (MS). Searches were complemented by consulting with experts in the field.
Study eligibility criteria targeted women who planned for a vaginal delivery or those who underwent an unplanned, emergency caesarean. All interventional and observational studies were deemed eligible.
Our primary endpoint was S-PPH, defined as a composite of blood loss of at least 1,000 mL, or a second-line treatment (uterine suture, pelvic vessel ligation, arterial embolization, hysterectomy), PPH-related blood transfusion, peripartum decrease in haemoglobin level, PPH-related maternal death. This outcome definition was selected because it was consistent with the WHO definition of S-PPH.1
Quality assessment and data extraction
The quality of studies was assessed using both the STROBE and CONSORT checklists, as appropriate for observational studies and RCTs.9,10
Data extraction was performed in duplicate using a standardized form by two independent researchers (TS and PG). Inter-observer discrepancies were again resolved by arbitration of the same third reviewer (MS).
Data analysis
Bias assessment
Bias analysis was adapted from both interventional and observational study designs. It was conducted with reference to the classic biases identified in the Cochrane Handbook or in Jean Bouyer’s epidemiology textbook (selection, performance, detection, attrition, classification, evaluation, and confusion biases).11,12 We rated biases in each study as “absent” (-), “possible” (+), “probable” (++), “highly probable” (+++), or “not applicable” (N/A).
Evaluation of risk factors for S-PPH
Risk factors for S-PPH were gauged using odds ratios (OR) and their 95% confidence interval (CI). Strength was defined for OR>2.0. They were classified as “key established” or of “uncertain significance” determinants using the Bradford Hill criteria’s framework for causal inference, under a pragmatic approach devised to comply with modern-day issues.13,14 Concretely, each of the factors identified through data extraction was gauged using the Bradford Hill criteria (strength, consistency, specificity, temporality, biological gradient, plausibility, coherence, experiment, and analogy) causality framework to determine the causative nature of the pathway between exposure to this factor and S-PPH. Causation and mediation were investigated using directed acyclic graphs, as done routinely in epidemiological practice.
Factors matching at least three of the abovementioned criteria, or two criteria while being linked to a known cause of bleeding (well-known aetiology of PPH, inherited or acquired haemorrhagic disease) were considered as “key established” determinants (involving the probability of a “causal pathway”, based on this abovementioned composite definition).
Factors matching one or two criteria without being linked to a known cause of bleeding were classified as of “associated uncertain status” and recommended for being explored in further study.
Factors accompanying development or progression of S-PPH, without matching any of the abovementioned criteria, nor being linked to a known cause of bleeding, were considered as indicators (or markers) of risk. By this, we mean factors indicating a risk of PPH without necessarily intervening in the causal chain between exposure and event.
RESULTS
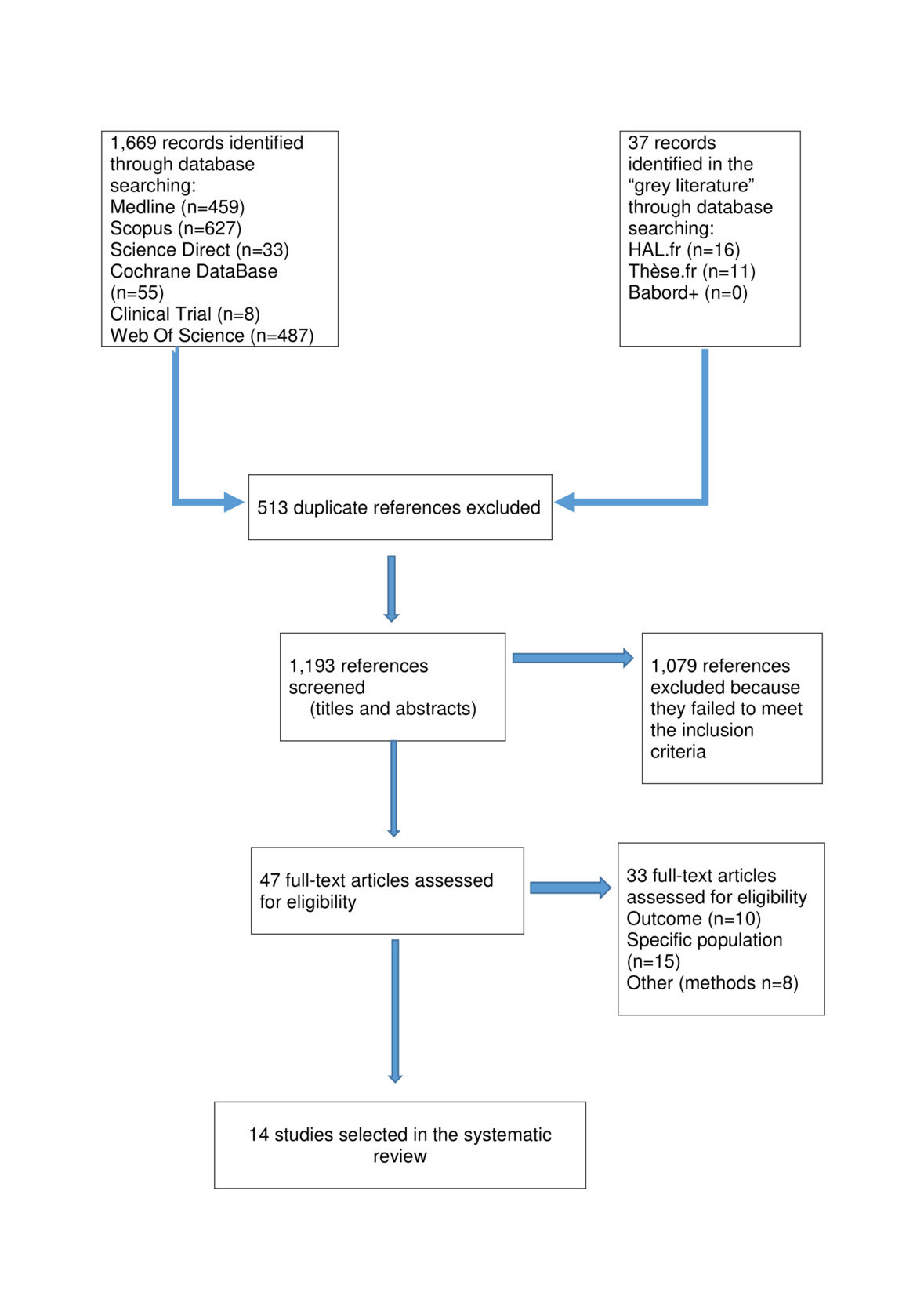
The selection process of the articles fulfilling the eligibility criteria for our systematic review is presented in Figure 1.
Selected studies
We identified 1,193 potentially relevant, non-duplicate studies. After reviewing the full text of these publications, we found a total of eleven articles that met our inclusion criteria.
Characteristics of selected studies
The descriptive characteristics of the retrieved studies15–28 including the period, location, study design, duration of enrolment, and the monocentric or multi-centric organisation of the fourteen selected studies, as well as the statistical method used for classifying risk factors in each of them, are summarized in Table 1.
The selected studies belonged to several study designs, as follows: cohort study (n=10), case-control study (n=3), and cluster randomized clinical trial (n=1). Inclusion periods ranged from ten months to nine years.
The total study population was composed of 9,271,519 parturient women (including 146,781 participants of the Pithagore-6 cluster randomized clinical trial). The number of cases of S-PPH was of 35,825 representing 3.9% of all childbirths.
In studies for which the proportion of S-PPH among all cases of PPH could be measured, the average proportion of S-PPH was of 25%. This figure differs slightly from the usually accepted ratio of one S-PPH for five PPH (20%).
The primary endpoint of each study fulfilled the outcome measure previously established in our review protocol. However, the definition of S-PPH varied across studies according to whether the selected outcome was single or composite.
Risk of bias in selected studies
Biases were independently evaluated for each study. Their analysis is summarized in Table 2.
For example, the study by Briley et al. was deemed to be potentially prone to a moderate selection bias given the small representativeness of this bicentric study and the use of secondary subsampling withdrawing the majority of PPH cases.17 Together with a 14% error rate in estimated blood loss, we considered it was also affected by a highly probable classification bias, while other biases were considered negligible.
Factors associated with S-PPH
The full assessment of risk factors using Bradford Hill’s criteria is displayed in Appendix S3 of the Online Supplementary Document.
Individual risk factors
Age
Six studies showed that maternal age of 35 years or above were associated with PPH in a significant, consistent, and coherent manner.15,17,24–27 In addition, Kramer et al. also showed a significant association between PPH and maternal age less than 19 years and the role of first pregnancy at older age on risk of S-HPP was highlighted.27 Indeed, the risk of S-PPH appears to be U-shaped, being higher before 19 and after 35 years.
Body Mass index
In the study by Briley et al., the body Mass Index (BMI) was associated with a linear increase of the S-PPH risk (OR 1.04, 95% CI=1.01-1.06, per one-unit increase).17 In the study by Nyfløt et al., obese women (BMI>30) were slightly more at risk of S-PPH (OR 1.29, 95% CI=1.02-1.63),16 while in turn, Paglia et al. found a higher risk of S-PPH when the parturient had a BMI less than 30 kg/m2.20
These seemingly contradictory results do not allow us to conclude about the risk’s direction between BMI and S-PPH.
Ethnicity
The risk of S-PPH was lower among patients from the Middle East (OR 0.60, 95% CI=0.45-0.81) but was higher among patients from Southeast Asia (OR 1.77, 95% CI=1.48-2.12) than it was among European patients.15 Another study found a strong risk (OR>2.0) of PPH in Hispanic populations (OR 3.14, 95% CI=1.23-9.32), as compared with Caucasians.20
Social context
The study by Briley et al. was the only to focus on the impact of patients’ neighbourhood, income level, and educational skills (these variables feeding an “index of multiple deprivation”) on PPH.17 Importantly, it showed that a disadvantaged neighbourhood was associated with an increased risk of severe PPH (OR 1.82, 95% CI=1.10-3.00).
Obstetrical history
Previous caesarean
The studies by Al Zirqi et al. (OR 1.46, 95% CI=1.02-2.20), Helman et al. (OR 2.75, 95% CI=1.42-5.33), and Kramer et al. (OR 1.3, 95% CI=1.2-1.3) found an association between a history of caesarean and S-PPH.15,18,27
These results are consistent, plausible, and coherent. They also met the criterion of temporality, according to which exposure precedes the effect. In agreement, a history of previous caesarean section was deemed a key established determinant of S-PPH.
Previous PPH
The strength of the association between a history of previous PPH and subsequent postpartum bleeding was consistent.16,17,22,24 Ditto, the criterion of temporality was met. In agreement, a history of previous PPH was deemed a key established determinant of S-PPH.
Parity
Al Zirqi et al. (OR 1.10, 95% CI=1.02-1.19), Driessen et al. (OR 1.88, 95% CI=1.51-2.33) and Dupont et al. (OR 1.71, 95% CI=1.43-2.05), reported an association between primiparity and S-PPH.15,22,24 It was less clear for multiparity. Given these discrepancies through parity, we concluded that regarding S-PPH, parity was a factor of uncertain significance.
Course of pregnancy
Multiple pregnancy
Eight studies showed a strong association between multiple pregnancy and severe bleeding.15–18,22–25 These results met the criteria of strength, consistency, plausibility, and coherence. Multiple pregnancy was considered as a key established determinant of S-PPH.
Embryonic annexes (placenta, amniotic membranes, umbilical cord)
Abnormal placentation led to S-PPH (OR 7.05, 95% CI=2.26-22.03).23 Kramer et al. confirmed this association with placenta praevia and placenta abruption (OR 7.00, 95% CI=6.60-7.30),27 though Marocchini et al. found a very strong association between placenta praevia and S-PPH (OR 22.00).25 In addition, Briley et al. specified that the risk of S-PPH was the higher with anterior placental presentation (OR 5.55, 95% CI=1.29-23.90).17 Kramer et al. showed a significant increase in the risk of PPH in the presence of polyhydramnios and chorioamnionitis.27
Given the strength, consistency, and plausibility of these results, we considered abnormal placentation as a key established determinant for S-PPH.
Pregnancy-induced hypertensive disorders
Although no association was found between preeclampsia and PPH, the strength and the dose-response relationship between pregnancy-induced hypertensive disorders and S-PPH found consistently in the literature support a causal association.17,20 Moreover, eclampsia complicated by a HELLP Syndrome (Haemolysis, Elevated Liver enzyme levels, Low Platelet count) was strongly and consistently associated with life-threatening haemorrhages.15,25
Given the coherence of these results, we classified preeclampsia as a key established determinant for S-PPH and low platelet counts as a key established indicator.
Uterine fibroids
Uterine fibroids increased the risk of PPH in the study by Nyfløt et al. (OR 2.51, 95% CI=1.36-4.64)16 and in the study by Kramer et al. (OR 2.00, 95% CI=1.80-2.20).27 Given uterine fibroids are known to cause bleeding in other circumstances (eg, menstruations), this association was found coherent, strong, and in accordance, uterine fibroids were considered as a key established obstetrical determinant of S-PPH.
Assisted reproduction
In vitro fertilization (IVF) or intra-cytoplasmic sperm injection (ICSI) increased the risk of S-PPH in the study by Nyfløt et al. (OR 2.72, 95% CI=1.93-3.85).16
Medications
Two studies assessed the impact of medications taken during pregnancy on S-PPH.17,20
The use of corticosteroids for foetal lung maturation was associated with an increased risk of S-PPH (OR 2.0, 95% CI=1.2-3.4).17 Magnesium injection, as used for alleviating preeclampsia, was also associated with S-PPH (OR 5.4, 95% CI=1.8-20.1).20 Both were deemed as indicators (markers of risk) of S-PPH.
Gestational age
An increased risk of S-PPH was shown for deliveries occurring before 37 weeks gestation (OR 5.0) (25), or after 41 weeks gestation (OR 1.30, 95% CI=1.08-1.56).24 Women whose infant was very small-for-gestational age (< 3rd percentile) were more likely to be transfused for S-PPH.25 These two studies did not allow us to draw a firm conclusion and this factor was classified as of uncertain significance.
Active phase of labour
Oxytocic drugs
Eight studies assessed the role of oxytocic drugs, used for labour induction, in S-PPH.15–18,20,24,27,28 Two was inconclusive,17,20 four of these showed a weak association (OR between 1.1 and 1.7),15,16,24,27 whereas two found a strong association (OR>2.0).18,28
The consistency, the coherence with current knowledge and temporality led us to consider labour induction as a key established determinant of S-PPH.
Labour duration
Prolonged active phase of labour, known to alter muscle contractility, was associated with an increased risk of S-PPH.15,16,22,24 In addition, Dupont et al. emphasised that bleeding risk was higher when the active phase of labour exceeded six hours (OR 1.70, 95% CI=1.143-2.01).24
Given the consistency and plausibility of these results, we concluded that prolonged labour is a key established determinant for S-PPH, without further confirming the six-hour time limit.
Uterine rupture
Uterine rupture, a complication that occurs mostly during the active phase of labour, has been recognised as cause of obstetric haemorrhage. The study by Kramer et al. confirmed a very strong association between uterine rupture and S-PPH (OR 11.6, 95% CI=9.7-13.8).27
Childbirth
Mode of delivery
Eight studies found that instrumental vaginal delivery and emergency caesarean were both associated with S-PPH.15–19,24,25,27
Given consistency and coherence of these results, we concluded that instrumental vaginal delivery and caesarean were key established determinants of S-PPH.
Foetal presentation
Kramer et al. showed that breech presentation increased the risk of S-PPH (OR 1.2, 95% CI=1.1-1.2).27 However, this single study did not allow us to draw a firm conclusion conducting this factor to be considered as of uncertain significance.
Delivery complications
Sosa et al. found a strong association between placenta retention and S-PPH (OR 16.0, 95% CI=7.1-36.0).28 Karlsson et al. highlighted a strong association between uterine exploration (usually an indicator of placenta retention) and risk of S-PPH (OR 23.0, 95% CI=14.3-37.1).19 These findings are highly coherent with current knowledge and recommendations.
Lesions in the birth canal
Cervical laceration was shown strongly associated with bleeding risk (OR 94.0, 95% CI=87.3-101.2).27
Together with the plausibility and coherence of these results, this strong association led us to conclude that if such a birth canal injury (cervical or vaginal laceration, episiotomy) was not sutured in the shortest time possible, blood loss would be substantial. In agreement, cervical injury was considered as a key established determinant of S-PPH.
Birthweight
Five studies an association between foetal macrosomia and the risk of S-PPH.15,16,24,27,28
The consistency and the coherence of these results with current knowledge led us to propose foetal macrosomia as a key established determinant of S-PPH, even though it may not be causal, this increasing the risk of other contributors on the causal pathway to S-PPH like, for instance, the lesions of birth canal.
Biological parameters
Low pre-pregnancy or low prepartum haemoglobin levels (<9 g/dL), prothrombin time (PT) below 50%, fibrinogen level below 2 g/L, and detectable level of Troponin-I before delivery, were considered as indicators of S-PPH.15,16,23
Epidural analgesia
Several studies examined the relationships between epidural analgesia during labour and S-PPH.17,22,24 Only two of these showed a weakly protective role (0.5<OR<1.0) of analgesia against S-PPH. Further studies are needed to draw a firm conclusion.
Management of severe PPH
Only four studies reported the impact of the medical management of labour on the incidence and prevalence of S-PPH.21,22,24,26
Importantly, Deneux-Tharaux et al. found no risk reduction in women exposed to a multifaceted intervention for reducing the incidence of S-PPH.21 In the Pithagore-6 cluster RCT similar incidence rates of S-PPH were observed in both the intervention arm (discussion of recommendations, reminders, audit) and the control group (passive diffusion of information). With respect to oxytocin injected during labour, one study found a protective effect when injected within 10 minutes.26 By contrast, Dupont et al. found an overall increased risk of S-PPH (OR 1.26, 95% CI=1.06-1.50),24 while the study by Driessen et al., within the same Pithagore6-trial framework, established that this risk was actual when given beyond 20 minutes after birth (OR 1.49, 95% CI=1.14-1.94).22 Given this inconsistency, oxytocin during labour was deemed as a factor of S-PPH of uncertain significance.
Summary of findings
We distinguished the factors associated with S-PPH into key established determinants, associated factors of uncertain significance, and indicators to guide the obstetrical decision. This classification is summarized in Table 3.
DISCUSSION
Based on a critical and pragmatic analysis of the literature, we propose that the key established determinants of S-PPH be identified, as follows: previous caesarean, previous PPH, multiple pregnancy, abnormal placentation, pregnancy-induced hypertensive disorders, labour induction, prolonged labour, uterine rupture, uterine fibroids, instrumental vaginal delivery and caesarean, uterine atony, placental retention, macrosomia and birth canal injuries. In addition, we require further studies to confirm the relevance of reproductive assistance technics (IVF/ICSI), epidural analgesia), gestational age, as well as social factors.
Together with the heterogeneity in the outcome measure definition, the very different study designs, population sizes, and definitions of exposure factors, prevented us to use meta-analysis for providing quantitative estimates of the associations between the candidate determinants and S-PPH.
Despite these limitations, we believe that the pragmatic approach that we used ensures internal validity of our results while minimising publication bias.
We confirm the primarily obstetrical nature of the key determinants of S-PPH. This observation fits current knowledge on the pathogenesis of postpartum bleeding.4,29 Among these, we distinguish causes and risk factors according to whether there are no or not mediators on the causal pathway linking exposure to severe bleeding. In agreement, uterine fibroids, abnormal placentation, preeclampsia, uterine rupture, uterus atony, placental retention, and birth canal injuries, are likely causes of PPH by different pathomechanisms *(*fibrinolysis and consumption of clotting factors) and should prompt active management to prevent evolution into S-PPH. By contrast, history of previous caesarean, history of previous PPH, multiple pregnancy, labour induction, prolonged labour, instrumental vaginal delivery and caesarean, require intermediate factors (abnormal placentation or uterus atony) are favouring conditions to severe bleeding in the third stage of labour or after delivery, which motivates additional explorations and interventions (e.g., timely reinjection of oxytocin,30 sublingual misoprostol,31 sulprostone,32 or early tranxenamic acid33) to contain blood losses.
Along with uncertain significance of their predictive value, the non-modifiable character of most socio-demographic factors does not support them for guiding obstetrical decisions.
However, the U-shaped curves of S-PPH risk observed both for maternal age and parity, should prompt caregiver’s awareness to young nulliparae and elderly grand multiparae at the top of the risk of S-PPH, while women with one single of these factors could be considered at an intermediate risk. Interestingly, these U-shaped curves are coherent with those observed in several other perinatal outcomes, such as preterm birth and small-for-gestational age for maternal age, but also with all-cause mortality later in life for parity.34
It is recognised that severely obese women with a pre-pregnancy BMI> 35 kg/m2 face multiple perinatal risks.35 Briley et al. have confirmed the existence of a positive association between maternal weight and S-PPH in a large prospective cohort.17 The evidence of a biological gradient between an increasing BMI and a higher risk of S-PPH likely supports causality. Actually, the finding suggest that maternal obesity has a modest effect on haemorrhage risk: the direction of the association may differ by delivery mode.36
The role of ethnicity in S-PPH is less clear and may differ according to both socio-environmental context and genetic factors. Briley et al. have suggested that Black African women were more likely to have PPH.17 In other studies, Southeast Asian15 and Hispanic20 women were the more at-risk of S-PPH, while Middle Eastern women seemed protected.15 Importantly, these ethnic groups at-risk have been confirmed recently in a large hospital-based study conducted in the US, in comparison to Non-Hispanic White, the lower-risk group, independently of confounding factors, including insurance status.37 Interestingly, among seven candidate genes, promoter polymorphism of the tissue factor gene (F3-603A>G) showed a significant association with PPH after adjustment for known risk factors, fuelling the idea of a genetic background that supports role of ethnicity in S-PPH.38 However, this hypothesis is challenged by another observation in low-income American women of increased risks of PPH with antidepressants uptake, independently of numerous confounders including race.39
During the last decade, the growing interest in reviews of clinical practices and the commitment to identifying suboptimal care has led to investigate the factors related to the management of PPH, both collectively and individually.
At maternity level, despite the inconclusive Pithagore-6 cluster RCT,21 community-based multifaceted interventions, aimed at improving the translation into clinical practices of the guidelines for PPH management, have gained attention in recent years and even proved effectiveness in low-middle income countries.40
At individual level, oxytocin infusion is mandatory for the prevention of third-stage labour and postpartum bleeding risks. In the presence of PPH, re-administration of oxytocin should be prompt, as it was shown ineffective when performed more than 20 min after onset of PPH to avoid progression towards S-PPH.22 In addition, caution should be the rule as treatment with oxytocin was associated with a harmful dose-response in a population-based nested case-control study (i.e., the higher the dose, the more severe the bleeding), which emphasises the need for more evidence-based indications and the minimal useful regimens.41
Epidural analgesia was found protective for the risk of S-PPH in two studies.22,24 This practice ensures comfort to parturient women and allows effective and rapid intervention in life-threatening obstetric emergencies. However, its benefits must be weighed against a potential decrease in uterine contractility, and hence against increased duration of labour, with expected difficulties in foetus expulsion.
We found four biological parameters, Troponin-I, haemoglobin, PT, and fibrinogen that seemed mere indicators of S-PPH as they are not clearly located on the causal pathway between exposure and disease. Among these, given that fibrinolysis may be important in S-PPH pathogenesis,22 only fibrinogen has paid attention so far, and multicenter trials testing the effectiveness of fibrinogen concentrates are currently underway.
Our systematic review has strengths and limitations. The attachment to the PRISMA statement throughout the different steps of this systematic review adds scientific rigour in the reporting of the results.
In the absence of consensual definition, we defined S-PPH as primary endpoint using the WHO definition. As a consequence, we excluded from our systematic review studies that used the former definition of S-PPH, such as Canadian and Italian studies.42,43
Our systematic review covers a wide range of geographic areas including North and South America,20,27,28 Southern and Northern Europe,15–17,19,21–25 West Africa26 and Israel.18 It gathers a very large population, which provides external validity and ensures extrapolations.
In a global context in which S-PPH remains the leading cause of maternal mortality, we rigorously gauged factors associated with S-PPH and distinguished key established determinants, as well as associated factors (especially contextual factors) and indicators that warrant further studies. We highlight socio-environmental factors and propose new studies to specify the impact of emerging factors on PPH, to take into account the context in which the disease occurs, and organise emergency obstetric care distribution and accessibility. In the field of obstetrical care, in which practices move faster than the pace of new publications, we endorse the concept of “living” systematic reviews and encourage regular updates of the current knowledge on S-PPH determinants combining rigour with timeliness.44
Acknowledgements
This work results of an internship of Master degree in clinical epidemiology. Tatiana Stachetti thanks the team of the CIC1410 for its warm welcoming during her internship, as well as Prof Laetitia Huiart, Head of the the CIC, for her supervision. The authors are indebted to Monsieur Jacques Gonthier, documentalist, to ease access to bibliography. Tatiana Stachetti also thanks Dr Marie Devred, gynaecologist-obstetrician, for her clinical expertise, guidance and proofreading. Patrick Gérardin thanks Dr Philippe Guillermin, gynaecologist-obstetrician, for furthering clinical research on PPH within the CIC. They also thank the teachers of the Master at the Institute of Public Health, Epidemiology and Development (ISPED), Bordeaux, especially Prof Rachid Salmi, Prof Rodolphe Thiébaut and Dr Karen Leffondre, for their precious advices as well as their colleagues for dedicated care to women’s health.
Funding
None.
Competing interests
The author completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author), and declare no conflicts of interest.
Correspondence to:
Madame Tatiana Stachetti
INSERM CIC1410
Clinical Epidemiology, Pôle Anesthésie
Centre Hospitalier Universitaire (CHU) Réunion
BP 350, 97448 Saint Pierre Cedex
Réunion
[email protected]
Dr Patrick Gérardin
INSERM CIC1410
Clinical Epidemiology
CHU de la Réunion
Groupe Hospitalier Sud Réunion
BP 350, 97448 Saint Pierre Cedex
Réunion
[email protected]